| View the data for Esperoct® (antihemophilic factor [recombinant], glycopegylated-exei). |
View online |
|
|
|
 |
| Esperoct® is indicated for on-demand treatment and control of bleeding episodes, perioperative bleed management, and routine prophylaxis in adults and children with hemophilia A. |
|
|
|
| Imagine your severe hemophilia A patients at mild levels. |
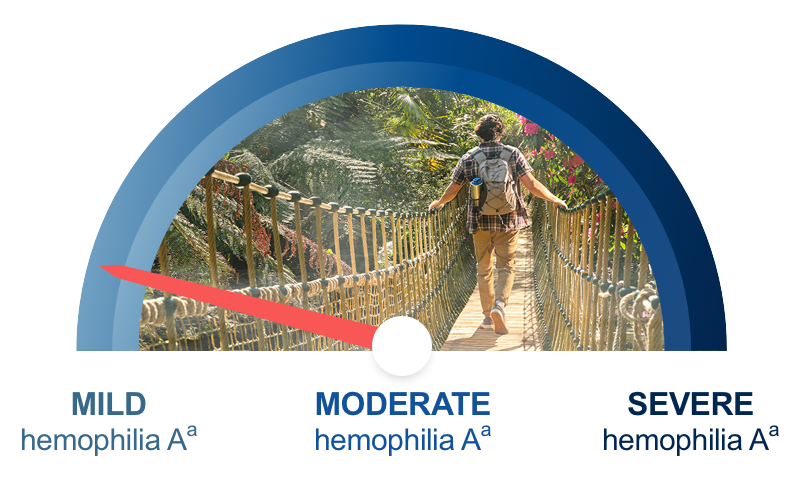 |
| Adolescents and adults with severe hemophilia A treated prophylactically with 50 IU/kg of Esperoct® every 3 to 4 days may be able to achieve factor levels associated with mild hemophilia A approximately 95% of the time based on PK modeling data.b |
|
|
aThe World Federation of Hemophilia defines the percentage of normal factor activity in blood in mild hemophilia as 5% - 40%; in moderate hemophilia as 1% - 5%; and of severe hemophilia as >1%.
bBased on PK modeling from PK assessments that were obtained from 8 previously treated patients (PTPs) aged ≥18 years with severe hemophilia A who participated in a Phase 1 open-label study, 23 PTPs aged ≥12 years with severe hemophilia A who participated in a Phase 3 open-label study, and 18 PTPs aged ≥18 years with severe hemophilia A who participated in a Phase 1 double-blind study; all received routine prophylaxis (50 IU/kg every 4 days). All PK assessments were based on a 50 IU/kg dose.1
|
| Keep scrolling for pharmacokinetic (PK) predictions for children <12. |

|
|
|
| Selected Important Safety Information |
| Contraindications |
| |
• |
Do not use in patients who have known hypersensitivity to Esperoct® or its
components, including hamster proteins
|
|
|
Warnings and Precautions
|
| |
• |
Hypersensitivity reactions, including anaphylaxis, may occur. Should
hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate
treatment
|
|
| Please click here or scroll below for additional Important Safety Information. |
|
|
| What if your pediatric patients could achieve moderate levels? |
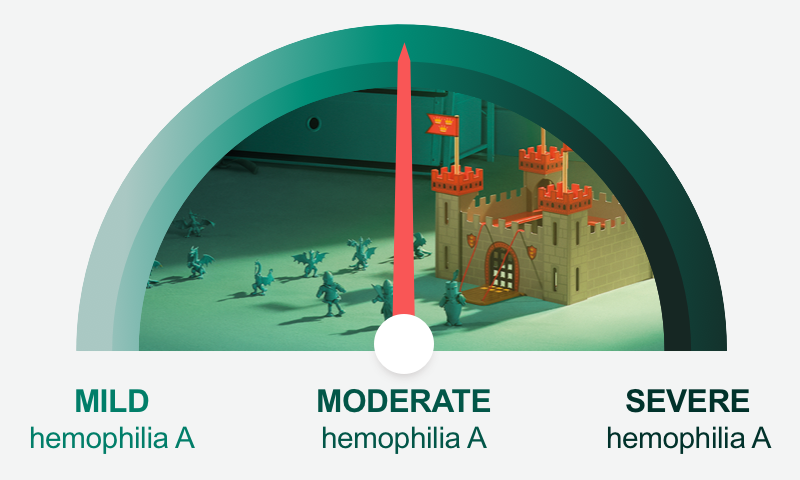 |
Children ages 0-11 years with severe hemophilia A treated prophylactically with ~60 IU/kg of Esperoct® every 3 to 4
days may be able to achieve factor levels associated
with moderate hemophilia A ~98% of the time.c |
|
|
| cBased on PK modeling from PK assessments that were obtained from 24 previously treated children (13 aged 0-5 years and 11 aged 6-11 years) with severe hemophilia A who participated in a Phase 3, open-label study; all received an average dose of approximately 60 IU/kg twice weekly. All PK assessments were based on a 50 IU/kg dose. |
|
| Have you signed up to receive Esperoct® updates? |
 |
Keep up with important information about Esperoct® and other treatments. |
 |
Receive the latest product news and information from Novo Nordisk. |
 |
Download, order, and share patient educational materials. |
|
|
|
|
|
|
| Indications and Usage |
|
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated
for use in adults and children with hemophilia A for on-demand treatment and control of bleeding
episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency
of bleeding episodes
|
| |
• |
Esperoct® is not indicated for the treatment of von Willebrand
disease
|
|
|
Important Safety Information (cont’d)
|
| Warnings and Precautions |
| |
• |
Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay
that measures Factor VIII inhibitor concentration if bleeding is not controlled with
the recommended dose of Esperoct® or if the expected plasma Factor
VIII activity levels are not attained
|
|
| Adverse Reactions |
| |
• |
The most frequently reported adverse reactions in clinical trials (≥1%) were
rash, redness, itching (pruritus), and injection site reactions
|
|
| Please click here for Prescribing Information. |
|
|
| References |
| |
1. |
Chowdary P, Carcao M, Holme P, et al. Fixed doses of N8-GP prophylaxis maintain moderate-to-mild Factor VIII levels in the majority of patients with severe hemophilia A. Res Pract Thromb Haemost. 2019;00:1-13. |
|
|
<%@ include view='hcpColoradoFooter' %>
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (e.g. name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
Esperoct® is a registered trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
© 2020 Novo Nordisk All rights reserved. US20ESP00078 July 2020 |
 |
|
|
|