
|
| Esperoct® is indicated for on-demand treatment and control of bleeding episodes, perioperative bleed management, and routine prophylaxis in adults and children with hemophilia A. |
|
|
| The latest clinical trial data further demonstrates effective prophylaxis. |
| Long-term trial results for Esperoct®—part of the largest and longest EHL clinical trial program for hemophilia A1—confirm effective prophylaxis. A lower median ABR was achieved compared with the main phases in the studies for children, adolescents, and adults.2-5,a,b |
|
| Esperoct® achieved a 0.8 median ABR (overall bleeds per year) in prophylaxis for pediatric patients ages 0 to <12 (n=68)c and for patients ages 12 to 70 years (n=177).d |
|
|
|
| EHL=extended half-life; ABR = Annual Bleed Rate. |
| aIn a phase 3, open-label study, safety, PK, and efficacy of Esperoct® were evaluated in PTPs aged ≥12 years with severe hemophilia A; 175 received routine prophylaxis (50 IU/kg every 4 days) and 12 adults elected to be treated on-demand during the main phase where a median ABR of 1.2 was achieved. After the main phase, a subset of patients continued on in extension phase part 1. After 24 weeks, patients from extension phase part 1 continued into the non-randomized extension part 2 until the end of trial.2,3 |
| bIn a phase 3 multinational, open-label, single-arm, non-randomized, non-controlled trial of 68 previously treated male patients aged <12 years old with severe congenital hemophilia A, comprising of a main phase and an extension phase. During the main phase, 68 children received prophylaxis at an average dose of approximately 65 IU/kg twice weekly for 26 weeks and a median ABR of 2.0 was achieved.4,5 |
| cMedian annualized bleeding rate shown is from the main and extension phase of previously treated children with severe hemophilia A, who took Esperoct® 60 IU/kg (50-75 IU/kg) twice weekly for a median of 5 years.5 |
| dMedian annualized bleeding rate shown is from the main and extension phases of the pivotal clinical trial of previously treated people aged ≥12 years with severe hemophilia A who received Esperoct® 50 IU/kg every 4 days, for up to 6.6 years.2 |
|
|
|
| Keep scrolling for more information about the extended half-life of Esperoct®. |
 |
|
| Selected Important Safety Information |
| Contraindications |
| |
• |
Do not use in patients who have known hypersensitivity to Esperoct® or its
components, including hamster proteins
|
|
|
Warnings and Precautions
|
| |
• |
Hypersensitivity reactions, including anaphylaxis, may occur. Should
hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate
treatment
|
|
|
Please
click here
or scroll below for additional Important Safety Information.
|
|
|
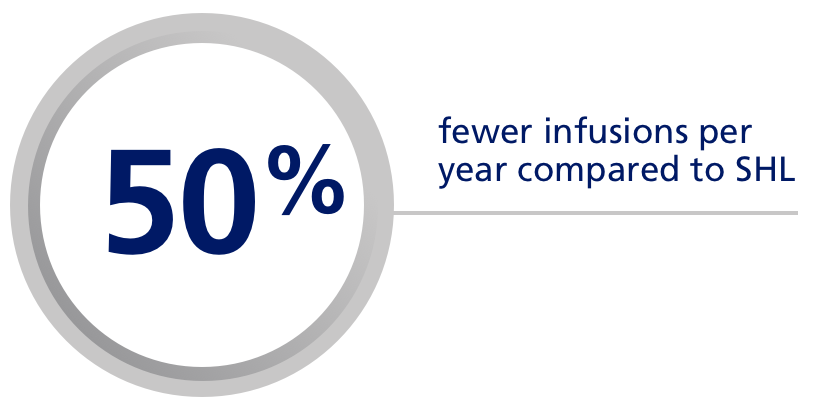
| Have your patients asked for fewer infusions? |
| With the extended half-life of Esperoct®, your adult and adolescent patients may need up to 50% fewer infusionse per year compared to standard half-life rFVIII treatments.6 |
 |
|
|
| e50% fewer if previously dosed every other day; 40% fewer if previously dosed 3x/week. |
|
| Have you created an account on the new novoMEDLINK™? |
 |
| Keep up with important information about bleeding disorders treatments. |
| Receive the latest bleeding disorders news from Novo Nordisk. |
| Download, order, and share educational materials for you and your patients. |
|
|
|
| Indications and Usage |
|
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated
for use in adults and children with hemophilia A for on-demand treatment and control of bleeding
episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency
of bleeding episodes
|
| |
• |
Esperoct® is not indicated for the treatment of von Willebrand
disease
|
|
|
Important Safety Information (cont’d)
|
| Warnings and Precautions |
| |
• |
Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay
that measures Factor VIII inhibitor concentration if bleeding is not controlled with
the recommended dose of Esperoct® or if the expected plasma Factor
VIII activity levels are not attained
|
|
| Adverse Reactions |
| |
• |
The most frequently reported adverse reactions in clinical trials (≥1%) were
rash, redness, itching (pruritus), and injection site reactions
|
|
| Please click here for Prescribing Information. |
|
|
References:
| 1. |
Esperoct® [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2019. |
| 2. |
Giangrande P, Andreeva T, Chowdary P, et al. Clinical evaluation of glycoPEGylated recombinant FVIII: efficacy and safety in severe haemophilia A. Thromb Haemost. 2017;117(2):252-261. |
| 3. |
Giangrande P, Karim F, Nemes L, et al. Long-term safety and efficacy of N8-GP in previously treated adults and adolescents with hemophilia A: Final results from pathfinder2. J Thromb Haemost. 2020;18(1):5-14. |
| 4. |
Meunier S, Alamelu J, Ehrenforth S, et al. Safety and efficacy of a glycoPEGylated rFVIII (turoctocog alpha pegol, N8-GP) in paediatric patients with severe haemophilia A. Thromb Haemost. 2017;117:1705-1713. |
| 5. |
Trakymiene SŠ, Economou M, Kenet G, et al. Long-term safety and efficacy of N8-GP in previously treated pediatric patients with hemophilia A: Final results from pathfinder5. J Thromb Haemost. 2020;18(1):15-25. |
| 6. |
Cafuir L and Kempton C. Current and emerging factor VIII replacement products for hemophilia A. Ther Adv Hem. 2017;8(10):303–313. |
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, the sponsor of this content, click here to unsubscribe. You also may call Novo Nordisk at 1.877.744.2579 or send a letter that includes your full contact information (e.g. name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see the Privacy Statement. |
|
Esperoct® is a registered trademark and novoMEDLINK™ is a trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
All other trademarks, registered or unregistered are the property of their respective owners.
© 2021 Novo Nordisk All rights reserved. US21ESP00047 June 2021 |
 |
|
|
|