| More patients stay on this rFVIII treatment |
View online |
|

|
Esperoct® is indicated for on-demand treatment and control of bleeding episodes, perioperative bleed management, and routine prophylaxis in adults and children with hemophilia A.
See limitations of use below. |
|
|
|
| |
| Patients who take Esperoct® are likely to stay with Esperoct® |
| Among extended half-life (EHL) products, fewer Esperoct® prescriptions were switched to another product.1,a |
|
 |
|
| aBased on data for Q2 2020-Q2 2021; accounts for net gains and losses of patients switching to and from extended half-life rFVIII available for at least one year. |
| Patients 12-70 years old: |
| Esperoct® achieves high trough levels in hemophilia A.2 |
|

|
for the entire dosing interval2,b
|
|

|
for 90% of the time in PK modeling data.2,c
|
|

|
mean trough levels maintained (n=61) as seen in 6+ year post-hoc analysis.3,d
|
|

|
mean trough levels maintained (n=61) as seen in 6+ years post-hoc analysis.3,d
|
|
|
|
| Esperoct® provides a low median annual bleed rate (ABR) across age groups.2,b |
| 0.8 ABR |
across all bleeds4
N=177 |
|
| Esperoct® has one proven starting dose with the option to individualize to meet patients' needs.2,5,e |
| 50 IU/kg |
|
every 4 days2,e
|
|
|
| bIn a phase 3, open-label study, safety, efficacy, and pharmacokinetics (PK) of Esperoct® were evaluated in PTPs aged >12 years with severe hemophilia A. Single-dose PK studies were performed in 42 adults after receiving Esperoct® 50 IU/kg; 175 PTPs received routine prophylaxis (50 IU/kg Q4D) for 76 weeks and 12 adults elected to be treated on demand during the main phase. Treatment-requiring bleeds were reported by patients through diaries. Mean trough levels for adolescents (12-<18 years) were 2.7 IU/dL.2,5 |
| cSteady-state FVIII activity profiles were estimated in 143 patients using a one-compartment model with first-order elimination with PK parameters of clearance and volume of distribution.2
|
| dExploratory descriptive analyses were used to evaluate long-term annual bleed rates and mean factor VIII trough levels which were assessed over time in 61 patients who received ≥6 years of prophylaxis, every 4 days. Limitations of the analyses include the lack of baseline joint status data, which is clinically relevant for phenotypic assessment prior to treatment initiation. The absence limits the ability to draw conclusions regarding improvement of joint status over time. Several trough-level data were excluded if it was believed that they were elevated due to dosing to treat a recent bleed.3
|
| eRegimen can be individually tailored to less or more frequent dosing based on bleeding episodes.2
|
|
|
|
|
|
| Scroll to see why Esperoct® may interest your standard half-life (SHL) hemophilia A patients. |
 |
|
| Selected Important Safety Information |
| Contraindications |
| |
• |
Do not use in patients who have known hypersensitivity to Esperoct® or its
components, including hamster proteins
|
|
|
Warnings and Precautions
|
| |
• |
Hypersensitivity reactions, including anaphylaxis, may occur. Should
hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate
treatment
|
|
| Please click here or scroll below for additional Important Safety Information. |
|
|
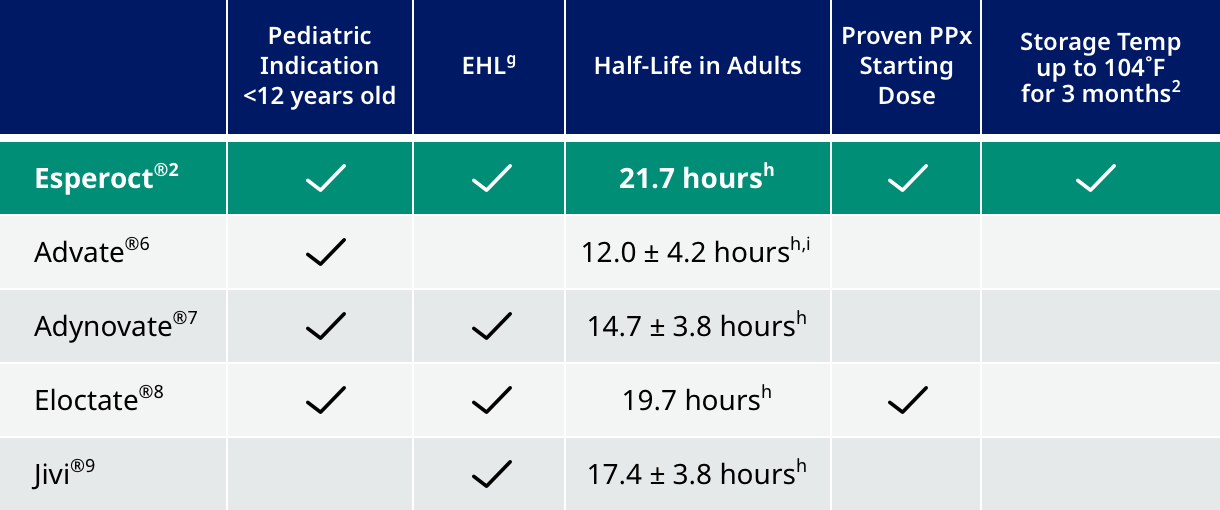
| See how Esperoct® compares to other rFVIII treatmentsf |
|
|
|
|
Not intended to be a comparison of efficacy or safety.
|
|
fData current as of April 2022
|
|
gEHL products are designed to extend time of FVIII in circulation by fusing another molecule to the FVIII, resulting in longer infusion intervals. Products in the EHL category were designed with half-life extension in mind, as opposed to FVIII products, which have slightly longer half-lives and may be prescribed twice weekly.
|
|
hBased on analysis of single dose PK using one-stage clotting assay in adult patients.
|
|
iPopulation is >16 years old.
|
|
|
|
|
|
Have you created an account on novoMEDLINKTM?
|

|
|
Keep up with important information about treatments for rare bleeding disorders.
|
|
Receive the latest news about rare bleeding disorders from Novo Nordisk.
|
|
Download, order, and share educational materials for you and your patients.
|
|
|
|
|
| Indications and Usage |
|
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated
for use in adults and children with hemophilia A for on-demand treatment and control of bleeding
episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency
of bleeding episodes
|
| |
• |
Esperoct® is not indicated for the treatment of von Willebrand
disease
|
|
|
Important Safety Information (cont’d)
|
| Warnings and Precautions |
| |
• |
Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay
that measures Factor VIII inhibitor concentration if bleeding is not controlled with
the recommended dose of Esperoct® or if the expected plasma Factor
VIII activity levels are not attained
|
|
| Adverse Reactions |
| |
• |
The most frequently reported adverse reactions in clinical trials (≥1%) were
rash, redness, itching (pruritus), and injection site reactions
|
|
| Please click here for Prescribing Information. |
|
|
References:
| 1. |
Data on file. Novo Nordisk Inc; Plainsboro, NJ. |
| 2. |
Esperoct [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2019. |
| 3. |
Tiede A, Hampton K, Jiménez-Yuste V, Young G, Benchikh El Fegoun S, Chowdry P. Post-hoc analysis on the long-term response to fixed-dose prophylaxis with N8-GP in patients with haemophilia A. Haemophilia. 2021;10.1111/hae.14409. |
| 4. |
Giangrande P, Karim F, Nemes L, et al. Long-term safety and efficacy of N8-GP in previously treated adults and adolescents with hemophilia A: Final results from pathfinder2. J Thromb Haemost. 2020;18(1):5-14. |
| 5. |
Giangrande P, Andreeva T, Chowdary P, et al. Clinical evaluation of glycoPEGylated recombinant FVIII: efficacy and safety in severe haemophilia A. Thromb Haemost. 2017;117(2):252-261. |
| 6. |
Advate [package insert]. Lexington, MA: Baxalta US Inc.; 2018 |
| 7. |
Adynovate [package insert]. Westlake Village, CA: Baxter Healthcare Corporation; 2021. |
| 8. |
Eloctate [package insert]. Cambridge, MA: Biogen Idec Inc.; 2020. |
| 9. |
Jivi [package insert]. Whippany, NJ: Bayer HealthCare LLC; 2018. |
|
|
<%@ include view='hcpColoradoFooter' %>
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (eg, name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
Esperoct® is a registered trademark of Novo Nordisk Heath Care AG.
novoMEDLINK™ is a trademark of Novo Nordisk A/S.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
All other trademarks, registered or unregistered are the property of their respective owners.
© 2022 Novo Nordisk All rights reserved. US22ESP00036 July 2022 |
 |
|
|
|