|
|

|
|
Esperoct® is used to treat and prevent or reduce the
number of bleeding episodes in people with hemophilia A.
|
|
|
|
|
Elevate your factor levels and reduce severity
|
 |
|
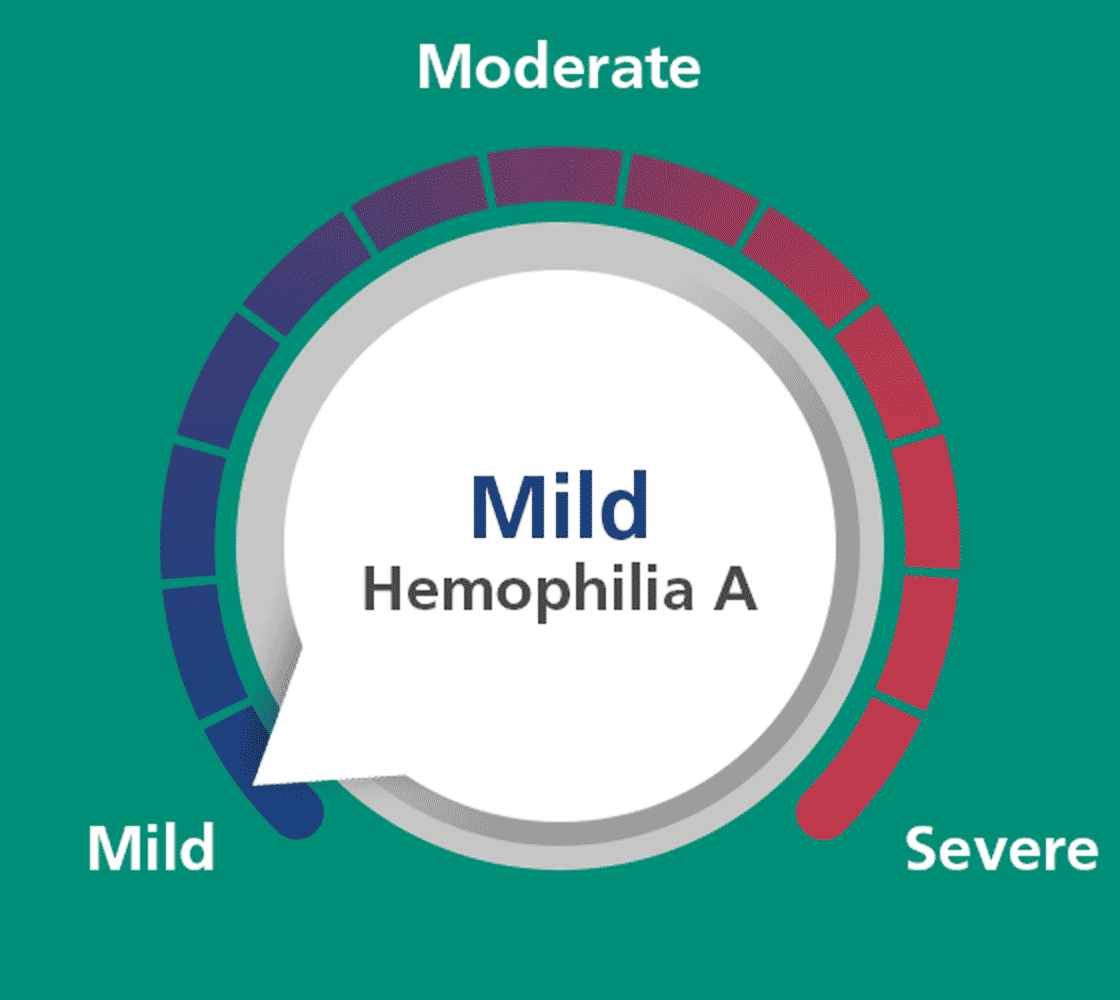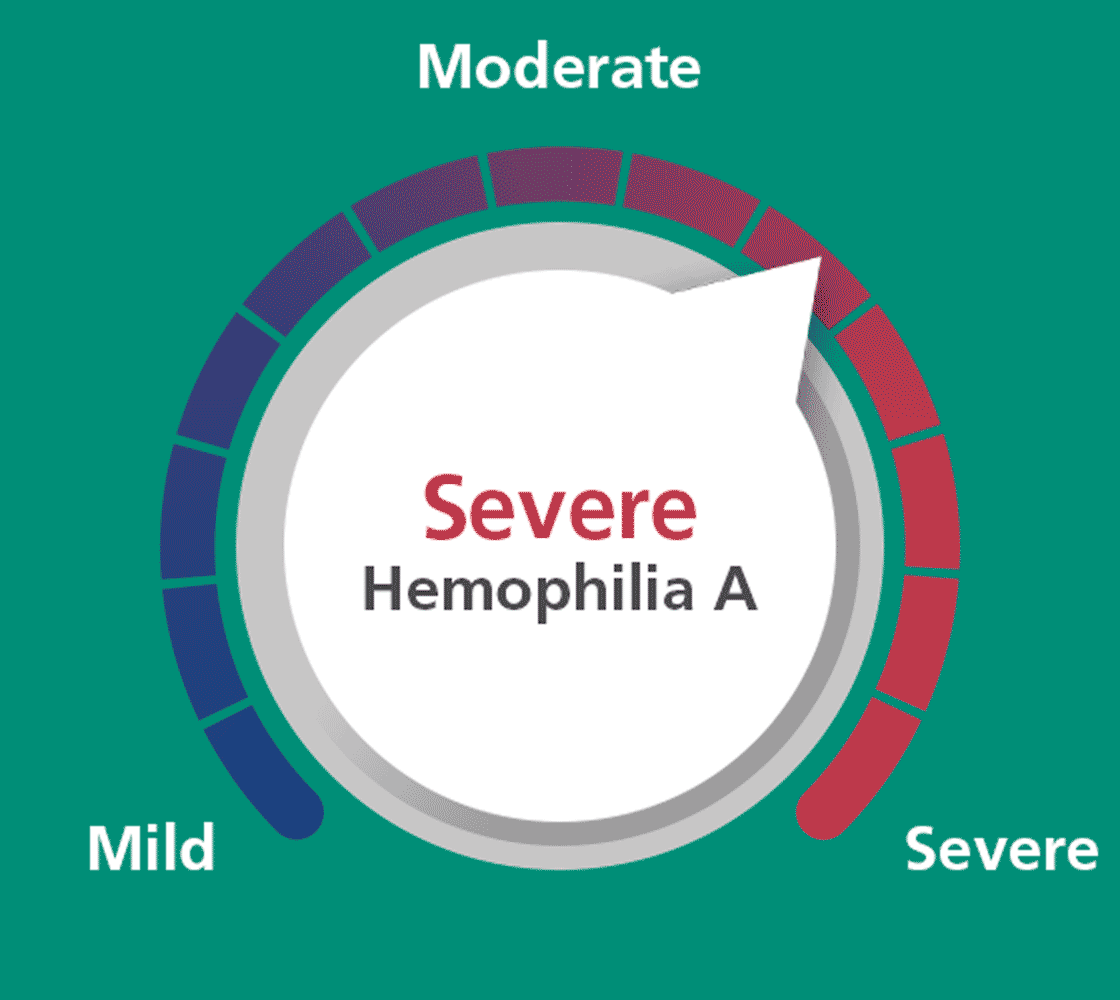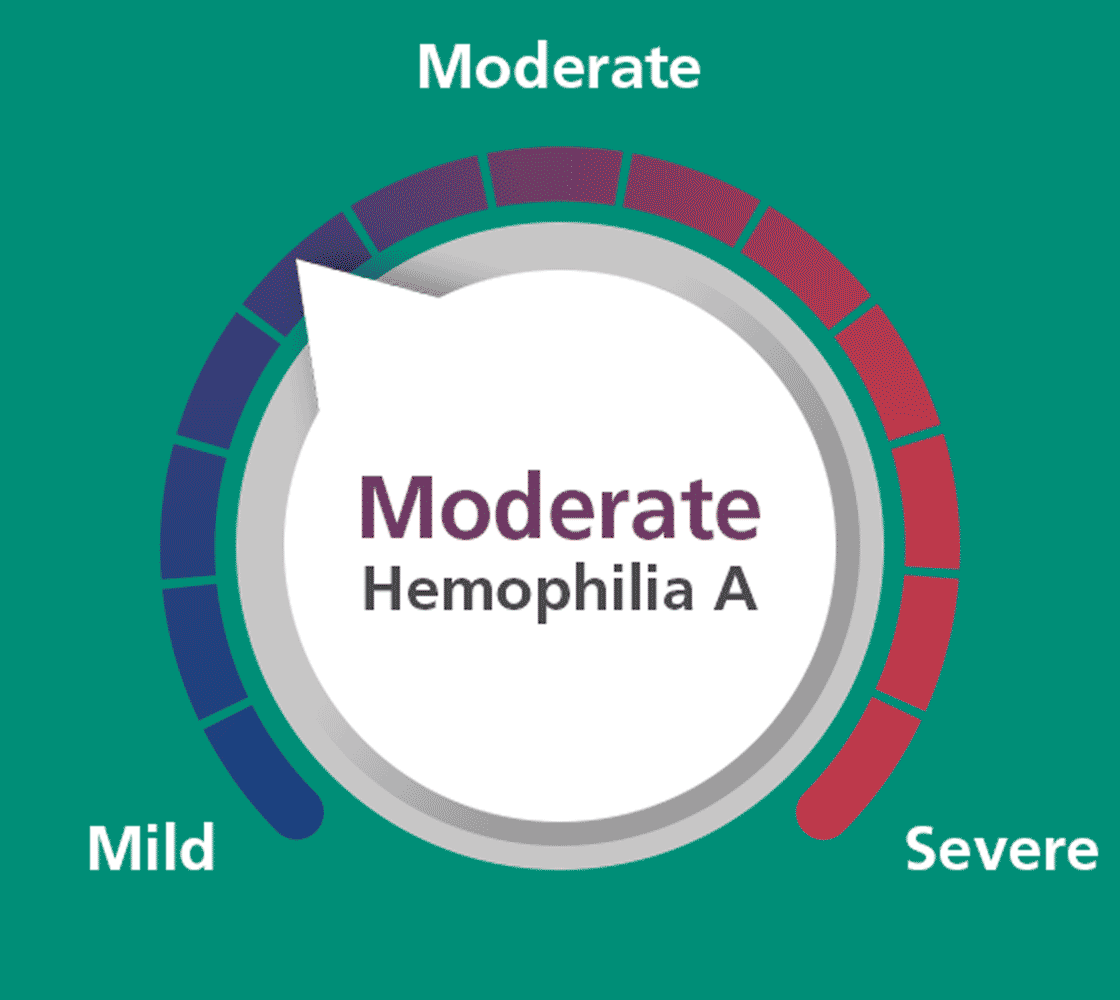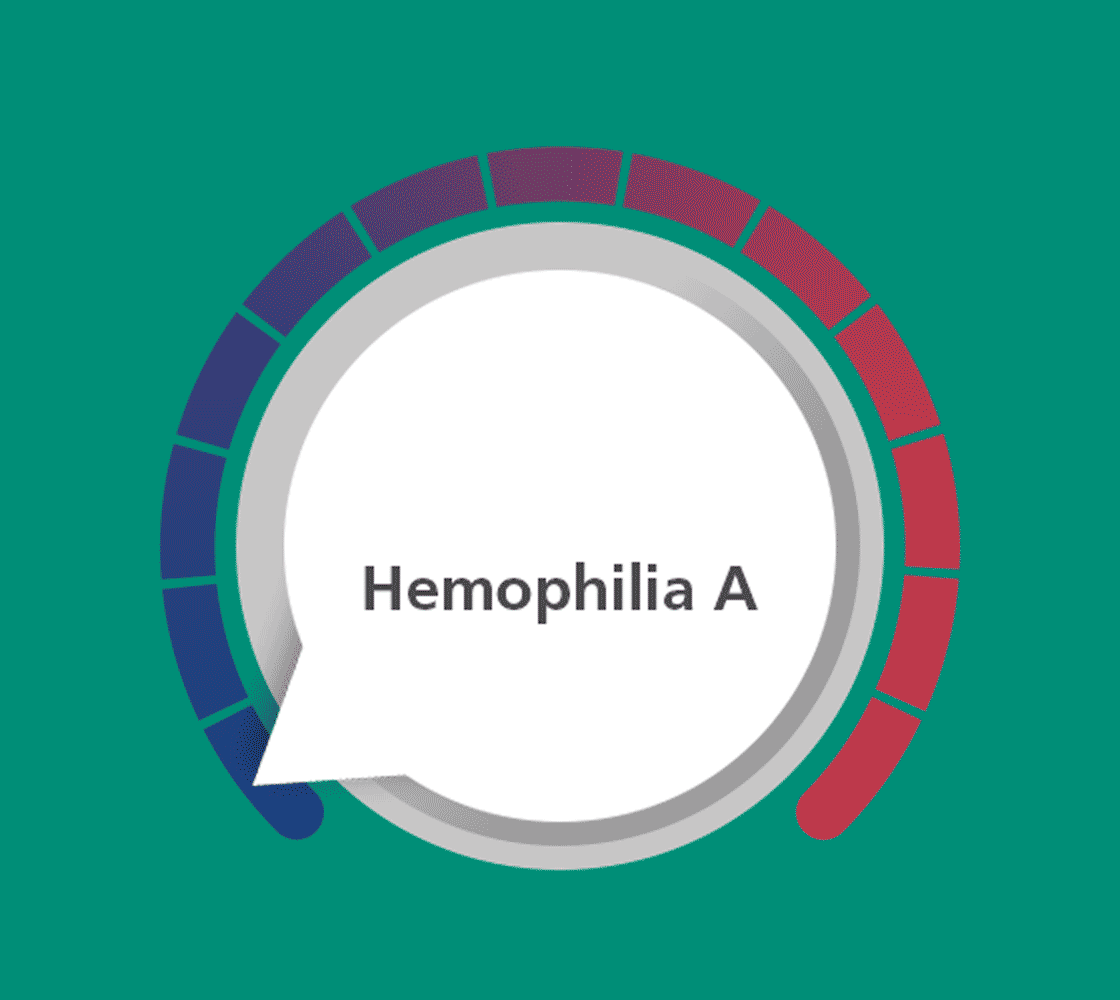
If you are an adult or adolescent with severe hemophilia A, treating with
Esperoct®
50 IU/kg every 4 days could help you
achieve factor levels defined as mild for 90% of the time and mild-to-moderate
for 100% of the time.a,b
|
|
|
|
|
|
a
|
Mild hemophilia A was defined as factor VIII (FVIII) activity >5 IU/dL (>5% of normal), moderate
as 1-5 IU/dL (1-5% of normal), and severe as <1 IU/dL (<1% of normal).
|
|
b
|
Steady-state factor VIII (FVIII) activity levels were estimated in 143 adults and
adolescents using PK modeling.
|
|
|
Scroll and learn how Esperoct® impacts children.
|

|
|
| Selected Important Safety Information |
| Who should not use Esperoct®? |
| |
• |
You should not use Esperoct® if you are allergic to factor VIII or any of the other ingredients of Esperoct® or if you are allergic to hamster proteins |
|
| What is the most important information I need to know about Esperoct®? |
| |
• |
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia treatment center |
|
| |
• |
Call your healthcare provider right away or get emergency treatment right away if you get any signs of an allergic reaction, such as: hives, chest tightness, wheezing, dizziness, difficulty breathing, and/or swelling of the face |
|
| Please click here or scroll below for additional Important Safety Information. |
|
|
|
From severe to mild: Esperoct® helps children too.
|
 |
|
With Esperoct® 60 IU/kg (50-75 IU/kg) twice weekly, children <12
years with severe hemophilia A could
achieve factor levels defined as mild for 72% of the time and mild-to-moderate
for 97.9% of the time.a,c
|
|
|
|
c
|
Steady-state factor VIII (FVIII) activity levels were estimated using PK
modeling in children <12 years. Twice weekly dosing was a dosing
interval of every 3 or 4 days.
|
|
|
|
| What is Esperoct®? |
|
Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is an
injectable medicine to treat and prevent or reduce the number of bleeding episodes in people
with hemophilia A. Your healthcare provider may give you Esperoct® when you have
surgery
|
| |
• |
Esperoct® is not used to treat von Willebrand Disease
|
|
| Important Safety Information (cont’d) |
|
What should I tell my healthcare provider before using Esperoct®?
|
| |
• |
Before taking Esperoct®, you should tell your healthcare provider if
you have or have had any medical conditions, take any medicines (including
non-prescription medicines and dietary supplements), are nursing, pregnant or
planning to become pregnant, or have been told that you have inhibitors to factor
VIII
|
| |
• |
our body can make antibodies called “inhibitors” against Esperoct®,
which may stop Esperoct® from working properly.
Call your healthcare provider right away if your bleeding does not stop after
taking Esperoct®
|
|
|
What are the possible side effects of Esperoct®?
|
| |
• |
Common side effects of Esperoct® include rash or itching, and
swelling, pain, rash or redness at the location of infusion
|
|
|
Please
click here
for Prescribing Information.
|
| Esperoct® is a prescription medication. |
|
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit
www.fda.gov/medwatch, or call 1-800-FDA-1088.
|
|
|
| PLEASE DO NOT RESPOND TO THIS EMAIL. If you would like to contact us, please click here or call 1‑877‑744‑2579. |
|
Esperoct® is a registered trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
© 2020 Novo Nordisk All rights reserved. US20ESP00119 January 2021 |
 |
|
|
|