|
|
 |
| Indicated for congenital hemophilia A or B with inhibitors, congenital Factor VII deficiency, Glanzmann’s thrombasthenia with refractoriness to platelets, and acquired hemophilia. See full indication below. |
|
|
|
Diagnosing and Treating Glanzmann’s Thrombasthenia (GT)
A NovoSeven® RT Educational Series for Healthcare Professionals |
| NovoSeven® RT: |
|
The only recombinant bypassing agent for patients with GT with refractoriness to platelets1,2
|
|
NovoSeven® RT is proven effective for treating GT-related bleeding episodes and for perioperative management in adults and children with refractoriness to platelet infusions, with or without antibodies to platelets.1,a
|
|
|
All bleeding episodes1,a
|
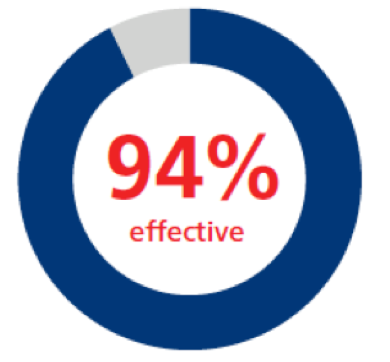
|
|
n=92; 266 bleeding events
|
|
|
All surgical procedures1,a
|
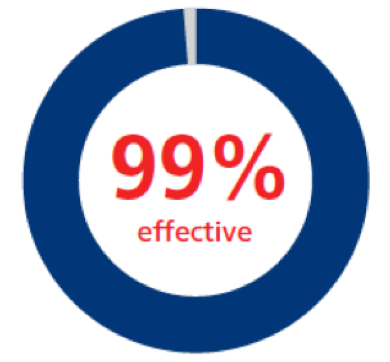
|
|
n=77; 160 bleeding events
|
|
|
|
aAdjudicator-assessed effectiveness of treatment regimens in patients with GT (N=218) in all severe bleeding episodes and all surgical procedures (N=1073) based on review of Glanzmann's Thrombasthenia Registry (GTR) data unblinded to investigator-coded efficacy. Efficacy was evaluated on a 2-point scale (clinical assessment of success or failure of treatment regimen as a whole, blinded and unblinded to investigator-coded outcome) including 92 patients treated with NovoSeven® RT for 266 bleeding episodes and 77 patients treated for 160 surgical procedures.
|
|
|
|
| Scroll to learn more about diagnosing GT. |
 |
|
| Selected Important Safety Information |
| WARNING: THROMBOSIS |
| • |
Serious arterial and venous thrombotic events following administration
of NovoSeven® RT have been reported
|
|
| • |
Discuss the risks and explain the signs and symptoms of thrombotic and
thromboembolic events to patients who will receive
NovoSeven® RT
|
|
| • |
Monitor patients for signs or symptoms of activation of the coagulation
system and for thrombosis
|
|
|
| Warnings and Precautions |
| • |
Serious arterial and venous thrombotic events have been reported in clinical trials
and postmarketing surveillance
|
|
| • |
Patients with congenital hemophilia receiving concomitant treatment with aPCCs
(activated prothrombin complex concentrates), older patients particularly with
acquired hemophilia and receiving other hemostatic agents, and patients with a
history of cardiac and vascular disease may have an increased risk of developing
thrombotic events
|
|
| Please click here or scroll below for additional Important Safety Information. |
|
|
|
|
Glanzmann's thrombasthenia (GT) can be easy to miss
|
|
|
|
GT shares symptoms with other acquired platelet disorders and von Willebrand disease, which can make it difficult to spot.3,4,c To help make sure your GT patients get the treatment they need without delay, use this diagnostic algorithm to learn more about identifying the signs and choosing a treatment.
|
|
|
|
cNovoSeven® RT is not indicated to treat von Willebrand disease.
|
|
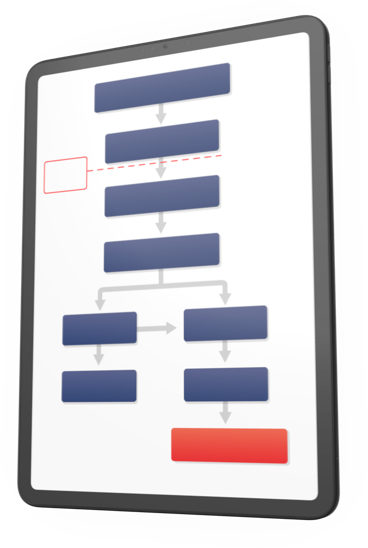
|
|
|
| Discover personalized professional education on novoMEDLINK™. |
 |
| If you found this information useful and would like to receive more curated educational updates and product information, create your account on novoMEDLINK™. |
|
|
|
|
| Indications and Usage |
|
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor
indicated for:
|
| • |
Treatment of bleeding episodes and perioperative management in adults and children
with hemophilia A or B with inhibitors, congenital Factor VII (FVII)
deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet
transfusions, with or without antibodies to platelets
|
|
| • |
Treatment of bleeding episodes and perioperative management in adults with acquired
hemophilia
|
|
| Important Safety Information (cont’d) |
| Warnings and Precautions |
| • |
Hypersensitivity reactions, including anaphylaxis, can occur with
NovoSeven® RT. Patients with a known hypersensitivity to mouse,
hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions.
Discontinue infusion and administer appropriate treatment when hypersensitivity
reactions occur
|
|
| • |
Factor VII deficient patients should be monitored for prothrombin time (PT) and
factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level,
or PT is not corrected, or bleeding is not controlled after treatment with the
recommended doses, antibody formation may be suspected and analysis for antibodies
should be performed
|
|
| • |
Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct
correlation to achieving hemostasis
|
|
| Adverse Reactions |
| • |
The most common and serious adverse reactions in clinical trials are thrombotic
events. Thrombotic adverse reactions following the administration of
NovoSeven® RT in clinical trials occurred in 4% of patients with
acquired hemophilia and 0.2% of bleeding episodes in patients with congenital
hemophilia
|
|
| Drug Interactions |
| • |
Thrombosis may occur if NovoSeven® RT is administered
concomitantly with Coagulation Factor XIII
|
|
|
Please click here for
Prescribing Information, including boxed Warning.
|
|
|
| 1. |
NovoSeven RT [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2020.
|
| 2. |
Poon MC, Di Minno G, d'Oiron R, et al. New insights into the treatment of Glanzmann thrombasthenia. Transfus Med Rev. 2016;30(2):92-99. |
| 3. |
Solh T, Botsford A, Solh M. Glanzmann's thrombasthenia: pathogenesis, diagnosis, and current and emerging treatment options. J Blood Med. 2015;6:219-227. |
| 4. |
Lambert MP. What to do when you suspect an inherited platelet disorder. Hematology Am Soc Hematol Educ Program. 2011;2011:377-383. |
|
|
<%@ include view='hcpColoradoFooter' %>
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (eg, name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
NovoSeven® is a registered trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
All other trademarks, registered or unregistered, are the property of their respective owners.
© 2022 Novo Nordisk All rights reserved. US22NSVN00063 October 2022 |
 |
|
|
|