| Take a look around the new novoMEDLINK™ experience. |
view online |
|
|
| a new way to access resources for rare bleeding disorders |
| |
|
|
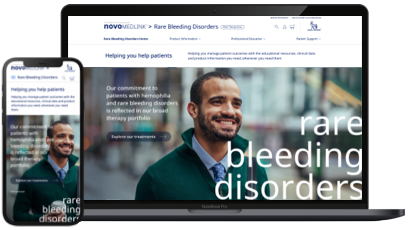
| All the resources you need, all in one place. |
| novoMEDLINK™ is now your go-to place for Novo Nordisk products, educational resources and disease state information to help patients with rare bleeding disorders and the people who treat them. |
 |
|
|
| A broad range of products for your patients |
| Novo Nordisk offers the following recombinant treatments for your appropriate patients with hemophilia and rare bleeding disorders: |
|
|
|
| awith refractoriness to platelets, with or without antibodies to platelets |
|
| Create your account on the new novoMEDLINK™ |
| On novoMEDLINK™, you can request trial prescriptions, order educational materials and access the latest product information across rare bleeding disorders. |
|
|
|
|
| Selected Important Safety Information for NovoSeven® RT |
| WARNING: THROMBOSIS |
| • |
Serious arterial and venous thrombotic events following administration of NovoSeven® RT have been reported |
|
| • |
Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive NovoSeven® RT |
|
| • |
Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis |
|
|
| Warnings and Precautions |
| • |
Serious arterial and venous thrombotic events have been reported in clinical trials and postmarketing surveillance |
|
| • |
Patients with congenital hemophilia receiving concomitant treatment with aPCCs (activated prothrombin complex concentrates), older patients particularly with acquired hemophilia and receiving other hemostatic agents, and patients with a history of cardiac and vascular disease may have an increased risk of developing thrombotic events |
|
|
Please click here or scroll below for additional NovoSeven® RT Important Safety Information.
|
|
| Indications and Usage |
|
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor
indicated for:
|
| • |
Treatment of bleeding episodes and perioperative management in adults and children
with hemophilia A or B with inhibitors, congenital Factor VII (FVII)
deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet
transfusions, with or without antibodies to platelets
|
|
| • |
Treatment of bleeding episodes and perioperative management in adults with acquired
hemophilia
|
|
| Important Safety Information (cont’d) |
| Warnings and Precautions |
| • |
Hypersensitivity reactions, including anaphylaxis, can occur with
NovoSeven® RT. Patients with a known hypersensitivity to mouse,
hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions.
Discontinue infusion and administer appropriate treatment when hypersensitivity
reactions occur
|
|
| • |
Factor VII deficient patients should be monitored for prothrombin time (PT) and
factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level,
or PT is not corrected, or bleeding is not controlled after treatment with the
recommended doses, antibody formation may be suspected and analysis for antibodies
should be performed
|
|
| • |
Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct
correlation to achieving hemostasis
|
|
| Adverse Reactions |
| • |
The most common and serious adverse reactions in clinical trials are thrombotic
events. Thrombotic adverse reactions following the administration of
NovoSeven® RT in clinical trials occurred in 4% of patients with
acquired hemophilia and 0.2% of bleeding episodes in patients with congenital
hemophilia
|
|
| Drug Interactions |
| • |
Thrombosis may occur if NovoSeven® RT is administered
concomitantly with Coagulation Factor XIII
|
|
| Please click here for NovoSeven® RT Prescribing Information including boxed Warning. |
|
|
| Selected Important Safety Information for Esperoct® |
| Contraindications |
| |
• |
Do not use in patients who have known hypersensitivity to Esperoct® or its
components, including hamster proteins
|
|
|
Warnings and Precautions
|
| |
• |
Hypersensitivity reactions, including anaphylaxis, may occur. Should
hypersensitivity reactions occur, discontinue Esperoct® and administer appropriate
treatment
|
|
| Please click here or scroll below for additional Esperoct® Important Safety Information. |
|
| Indications and Usage |
| Esperoct® [antihemophilic factor (recombinant), glycopegylated-exei] is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes |
| |
• |
Esperoct® is not indicated for the treatment of von Willebrand
disease
|
|
|
Important Safety Information (cont’d)
|
| Warnings and Precautions |
| |
• |
Development of neutralizing antibodies (inhibitors) has occurred. Perform an assay
that measures Factor VIII inhibitor concentration if bleeding is not controlled with
the recommended dose of Esperoct® or if the expected plasma Factor
VIII activity levels are not attained
|
|
| Adverse Reactions |
| |
• |
The most frequently reported adverse reactions in clinical trials (≥1%) were
rash, redness, itching (pruritus), and injection site reactions
|
|
| Please click here for Esperoct® Prescribing Information. |
|
|
| Selected Important Safety Information for Rebinyn® |
| Contraindications |
| • |
|
Rebinyn® is contraindicated in patients with a known hypersensitivity to Rebinyn® or its components, including hamster proteins. |
|
| Warnings and Precautions |
| Hypersensitivity reactions, including anaphylaxis, may occur. Signs may include angioedema, chest tightness, difficulty breathing, wheezing, urticaria, and itching. Discontinue Rebinyn® if allergic or anaphylactic type reactions occur and initiate appropriate treatment. |
| Please click here or scroll below for additional Rebinyn® Important Safety Information. |
|
| Indications and Usage |
| Rebinyn®, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B for on demand treatment and control of bleeding episodes and perioperative management of bleeding. |
| Limitations of Use: Rebinyn® is not indicated for routine prophylaxis or for immune tolerance induction in patients with hemophilia B. |
| Important Safety Information (cont’d) |
| Warnings and Precautions |
| • |
|
Development of neutralizing antibodies (inhibitors) to Factor IX may occur. Monitor patients for development of Factor IX inhibitors if bleeding is not controlled with the recommended dose of Rebinyn® or if expected Factor IX activity plasma levels are not attained. Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used. |
|
| • |
|
The use of Factor IX-containing products has been associated with thrombotic complications. Monitor for thrombotic and consumptive coagulopathy when administering Rebinyn® to patients with liver disease, post-operatively, to newborn infants, or to patients at risk of thrombosis or disseminated intravascular coagulation (DIC). |
|
| • |
|
Nephrotic syndrome has been reported following immune tolerance induction therapy with Factor IX products in hemophilia B patients with Factor IX inhibitors, often with a history of allergic reactions to Factor IX. The safety and efficacy of using Rebinyn® for immune tolerance induction have not been established. |
|
| Adverse Reactions |
| • |
|
The most common adverse reactions reported in clinical trials (≥1%) were itching and injection site reactions. |
|
| • |
|
Animals administered repeat doses of Rebinyn® showed accumulation of PEG in the choroid plexus. The potential clinical implications of these animal findings are unknown. |
|
| Please click here for Rebinyn® Prescribing Information. |
|
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (e.g. name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
Esperoct®, NovoSeven® and Rebinyn® are registered trademarks and novoMEDLINK™ is a trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
© 2021 Novo Nordisk All rights reserved. US21NSVN00081 December 2021 |
 |
|
|
|
|