|
|
 |
| Indicated for congenital hemophilia A or B with inhibitors, congenital Factor VII deficiency, Glanzmann’s thrombasthenia with refractoriness to platelets, and acquired hemophilia. See full indication below. |
|
|
|
| Success Speaks for Itself | Hospital Series |
| When it comes to breakthrough bleeds, every minute counts. |
|
| When congenital hemophilia with inhibitors (CHwI) patients of all ages experience acute bleeds, treating right away may minimize short and long-term impacts.1 Real-world evidence from a post-hoc subgroup analysis of SMART-7 data showed that treating acute bleeds rapidly with NovoSeven® RT resulted in higher hemostatic efficacy for CHwI patients.2 |
|
| Within 1 hour2,a,b |
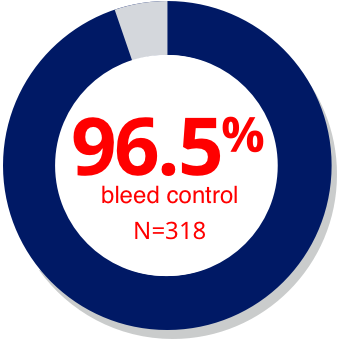 |
|
|
| > 4 hours2,a,b |
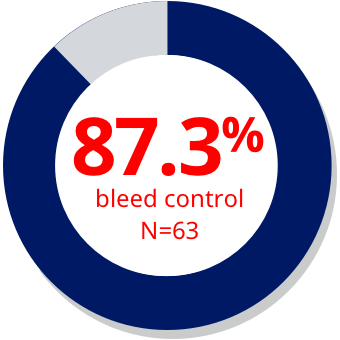 |
|
|
| aData from a prospective, observational, single-arm, open-label study (SMART-7) in patients with hemophilia A or B evaluating safety and effectiveness of the room temperature stable formulation of rFVIIa (NovoSeven® RT) (n=45). Hemostatic response following rFVIIa monotherapy by time to first treatment in different age cohorts was a post-hoc, subgroup analysis. Patients evaluated the status of bleeding episodes after each treatment as “bleed stopped,” “bleed slowed,” or “no change/worsened.” Based on these patient evaluations, treatment was described as “effective,” “partially effective,” or “ineffective” respectively.2 |
| bThe median (min; max) time from bleed start to first dose for the bleeding episodes (n=482) treated with rFVIIa monotherapy in SMART-7™ was 30 minutes (0.0; 10,335), indicating that the majority of bleeds were treated quickly. The majority of bleeding episodes treated with rFVIIa monotherapy (318/482 [66%]) were treated within 1 hour. |
|
|
|
| Selected Important Safety Information |
| WARNING: THROMBOSIS |
| • |
Serious arterial and venous thrombotic events following administration
of NovoSeven® RT have been reported
|
|
| • |
Discuss the risks and explain the signs and symptoms of thrombotic and
thromboembolic events to patients who will receive
NovoSeven® RT
|
|
| • |
Monitor patients for signs or symptoms of activation of the coagulation
system and for thrombosis
|
|
|
| Warnings and Precautions |
| • |
Serious arterial and venous thrombotic events have been reported in clinical trials
and postmarketing surveillance
|
|
| • |
Patients with congenital hemophilia receiving concomitant treatment with aPCCs
(activated prothrombin complex concentrates), older patients particularly with
acquired hemophilia and receiving other hemostatic agents, and patients with a
history of cardiac and vascular disease may have an increased risk of developing
thrombotic events
|
|
| Please click here or scroll below for additional Important Safety Information. |
|
|
|
 |
| MASAC recommends rFVIIa as a first-line treatment for acute bleeds in CHAwI patients taking Hemlibra®.3 NovoSeven® RT is the only rFVIIa indicated for use with CHwI patients of all ages4 and proven safe to use with Hemlibra for CHAwI patients.5 |
| MASAC=Medical and Scientific Advisory Council, National Hemophilia Foundation |
| CHAwI = congenital hemophilia A with inhibitors |
|
|
|
| Rely on NovoSeven® RT to quickly and effectively treat bleeds across age groups4,6 |
| NovoSeven® RT delivered 98% effective bleed control in patients ≤18 years, based on real-world experience.7,c One of the largest clinical trials was conducted in patients with CHwI and evaluated NovoSeven® RT in joint, target joint, mucocutaneous, muscle, and other bleeding episodes.8 |
| cA retrospective analysis evaluated bleeding episodes among 429 inhibitor patients treated with rFVIIa recorded in the HTRS Registry. Treatment response was classified into four categories: bleeding stopped; bleeding stopped but re-bleeding within 48 hours; bleeding slowed but not stopped; or bleeding did not stop. Effectiveness is defined as bleeding stopped or slowed. |
|
|
|
| Have you created an account on novoMEDLINK™? |
 |
| Keep up with important information about treatments for rare bleeding disorders. |
| Receive the latest news about rare bleeding disorders from Novo Nordisk. |
| Download, order, and share educational materials for you and your patients. |
|
|
|
|
| Indications and Usage |
|
NovoSeven® RT (coagulation Factor VIIa, recombinant) is a coagulation factor
indicated for:
|
| • |
Treatment of bleeding episodes and perioperative management in adults and children
with hemophilia A or B with inhibitors, congenital Factor VII (FVII)
deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet
transfusions, with or without antibodies to platelets
|
|
| • |
Treatment of bleeding episodes and perioperative management in adults with acquired
hemophilia
|
|
| Important Safety Information (cont’d) |
| Warnings and Precautions |
| • |
Hypersensitivity reactions, including anaphylaxis, can occur with
NovoSeven® RT. Patients with a known hypersensitivity to mouse,
hamster, or bovine proteins may be at a higher risk of hypersensitivity reactions.
Discontinue infusion and administer appropriate treatment when hypersensitivity
reactions occur
|
|
| • |
Factor VII deficient patients should be monitored for prothrombin time (PT) and
factor VII coagulant activity (FVII:C). If FVII:C fails to reach the expected level,
or PT is not corrected, or bleeding is not controlled after treatment with the
recommended doses, antibody formation may be suspected and analysis for antibodies
should be performed
|
|
| • |
Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct
correlation to achieving hemostasis
|
|
| Adverse Reactions |
| • |
The most common and serious adverse reactions in clinical trials are thrombotic
events. Thrombotic adverse reactions following the administration of
NovoSeven® RT in clinical trials occurred in 4% of patients with
acquired hemophilia and 0.2% of bleeding episodes in patients with congenital
hemophilia
|
|
| Drug Interactions |
| • |
Thrombosis may occur if NovoSeven® RT is administered
concomitantly with Coagulation Factor XIII
|
|
|
Please click here for
Prescribing Information, including boxed Warning.
|
|
|
| 1. |
Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1-158. |
| 2. |
Demartis F, Batorova A, Chambost H, et al. Real-world early treatment with room temperature-stable recombinant Factor VIIa in hemophilia A/B and inhibitors: SMART-7™ post hoc analysis. TH Open. 2017;1(2):e130-e138. |
| 3. |
National Hemophilia Foundation. Recommendation on the use and management of emicizumab-kxwh (hemlibra®) for hemophilia a with and without inhibitors, #268. New York, NY: National Hemophilia Foundation; 2022. |
| 4. |
NovoSeven® RT [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2020. |
| 5. |
Levy GG, Asikanius E, Kuebler P, et al. Safety analysis of rFVIIa with emicizumab dosing in congenital hemophilia A with inhibitors: experience from the HAVEN clinical program. J Thromb Haemost. 2019;17(9):1470-1477. |
| 6. |
Bysted BV, Scharling B, Moller T, Hansen BL. A randomized, double-blind trial demonstrating bioequivalence of the current recombinant activated factor VII formulation and a new robust 25°C stable formulation. Haemophilia. 2007;13(5):527-532. |
| 7. |
Neufeld EJ, Saxena K, Kessler CM, et al. Dosing, efficacy, and safety of recombinant factor VIIa (rFVIIa) in pediatric versus adult patients: the experience of the Hemostasis and Thrombosis Research Society (HTRS) Registry (2004-2008). Pediatr Blood Cancer. 2013;60(7):1178-1183. |
| 8. |
Lentz SR, Ehrenforth S, Abdul Karim F, et al; adept™2 investigators. Recombinant factor VIIa analog in the management of hemophilia with inhibitors: results from a multicenter, randomized, controlled trial of vatreptacog alfa. J Thromb Haemost. 2014;12(8):1244-1253. |
|
|
<%@ include view='hcpColoradoFooter' %>
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (eg, name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
NovoSeven® is a registered trademark of Novo Nordisk Health Care AG.
novoMEDLINK™ is a trademark of Novo Nordisk A/S.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
All other trademarks, registered or unregistered, are the property of their respective owners.
© 2022 Novo Nordisk All rights reserved. US22NSVN00039 August 2022 |
 |
|
|
|