| This Factor IX therapy can help your patients achieve higher PK levels for longer. |
View online |
|
|
 |
| Indicated for use in adults and children with hemophilia B for on-demand treatment and control of bleeding episodes, perioperative management of bleeding. |
|
|
|
| Your patients could stay in the non-hemophilia range for longer |
|
|
|
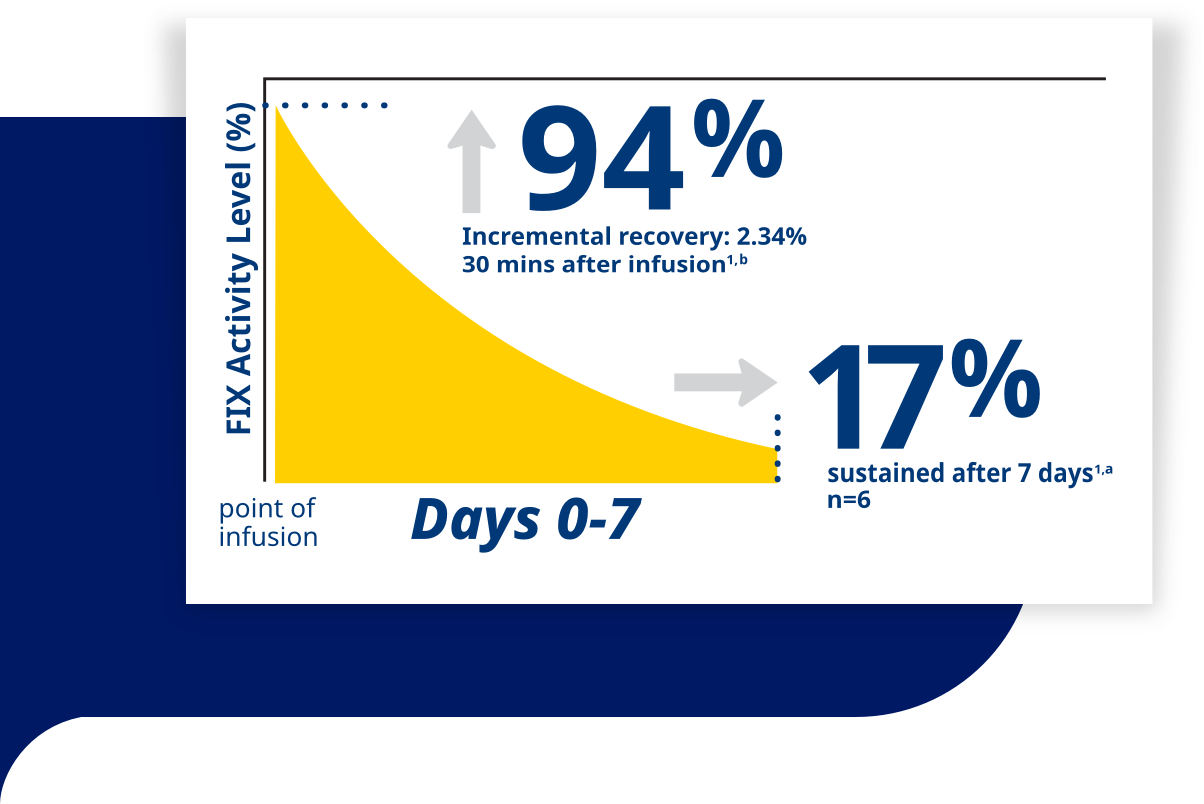
| The high incremental recovery of Rebinyn® boosts Factor IX (FIX) levels into a range that, when combined with the extended half-life, allows for prolonged periods in the non-hemophilia range.1,2,a |
|
|
|
|
| A single dose of Rebinyn® (40 IU/kg) achieved a 94% increase in FIX activity level and a 2.34% incremental recovery 30 minutes after infusion.1,a,c |
|
| |
 |
| |
| Rebinyn® achieved an 83-hour half-life in adults.1,a |
|
|
|
aBased on pharmacokinetic (PK) assessment of a single dose of Rebinyn® 40 IU/kg in 6 adults (mean FIX activity after 7 days 16.8%), 3 adolescents (mean FIX activity 14.6%), 13 children aged 7-12 (mean FIX activity 10.9%), and 12 children aged 0-6 (mean FIX activity 8.4%) upon enrollment in the phase 3 trials using 1-stage assay and product specific standard. All values are geometric mean.
bBased upon a 2.34% increase in factor levels per IU/kg infused in adults.1
cFactor levels were elevated above baseline in adults with factor levels ≤2%. |
|
| Scroll to see comparative data. |
 |
|
| Selected Important Safety Information |
| Contraindications |
| • |
|
Rebinyn® is contraindicated in patients with a known hypersensitivity to Rebinyn® or its components, including hamster proteins. |
|
| Warnings and Precautions |
| Hypersensitivity reactions, including anaphylaxis, may occur. Signs may include angioedema, chest tightness, difficulty breathing, wheezing, urticaria, and itching. Discontinue Rebinyn® if allergic or anaphylactic type reactions occur and initiate appropriate treatment. |
| Please click here or scroll below for additional Important Safety Information. |
|
|
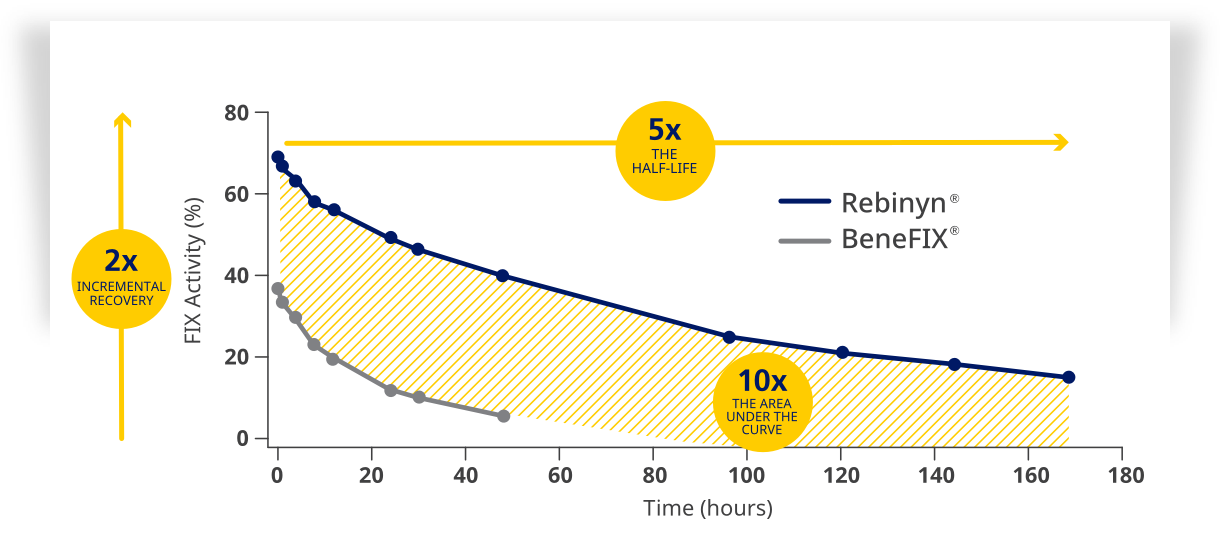
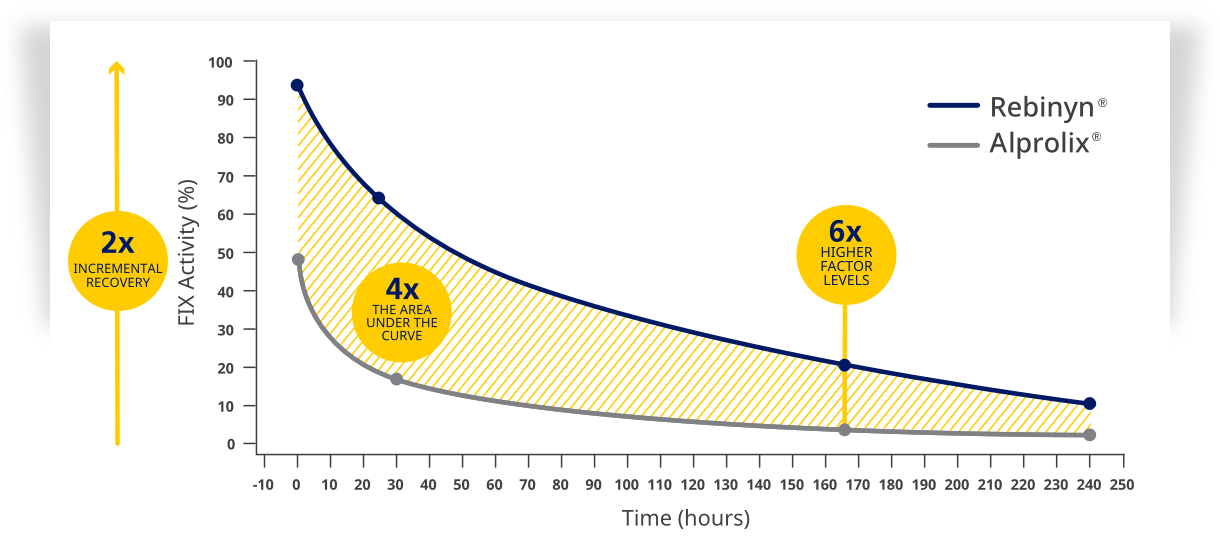
| Higher factor levels for longer, compared to BeneFIX® and Alprolix® |
| Rebinyn® has an improved PK profile compared to BeneFIX® and Alprolix®. Rebinyn® has higher incremental recovery, longer half-life, and greater area under the curve than BeneFIX® and Alprolix®.1,3,4,a,c |
| Rebinyn® achieved and maintained a higher factor activity than BeneFIX® and Alprolix®1,4,d,e,f,g |
|
| Comparison to BeneFIX® |
|
| Comparison to Alprolix® |
|
|
| • |
Rebinyn® is not approved for routine prophylaxis |
| • |
Animals given repeat doses of Rebinyn® showed accumulation of PEGylation in the choroid plexus. The potential clinical implications of these animal findings are unknown |
|
|
|
| dBased upon a phase 1 study of patients administered one of three doses of Rebinyn® (25, 50, or 100 IU/kg) compared with one 50 IU/kg dose of their prior SHL rFIX (n=7) or pdFIX (n=8) using a one-stage assay and product-specific standard. For Rebinyn®, estimated mean activity is adjusted to a dose of 50 IU/kg to allow for comparison of dose-dependent parameters. Differences were similar in comparison of Rebinyn® to pdFIX recovery 1.3 vs 1.1 IU/dL per IU/kg, 1.2x; half-life 93 vs 18 hours, 5.2x; AUC 70 vs 9 U x h/mL, 8x).3 |
| eBased upon a phase 1 study of 15 patients administered a single dose of Rebinyn® 50 IU/kg compared with a single dose of Alprolix 50 IU/kg using both 1-stage (shown above) and chromogenic assays. The standard Alprolix dose of 50 IU/kg was administered for both products to allow for comparison of dose-dependent parameters; dose normalized to 50 IU/kg to reflect minor differences in dose administered. Geometric mean half-life was also prolonged (Rebinyn®: 103.2 hours, Alprolix: 84.9 hours). All comparisons were significant (P<0.0001) for all assays.4 |
| fIU x h/mL. |
| gPer IU/kg. |
|
|
| Have you created an account on novoMEDLINK™? |
 |
| Keep up with important information about treatments for rare bleeding disorders. |
| Receive the latest news about rare bleeding disorders from Novo Nordisk. |
| Download, order, and share educational materials for you and your patients. |
|
|
|
|
| Indications and Usage |
| Rebinyn®, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B for on demand treatment and control of bleeding episodes and perioperative management of bleeding. |
| Limitations of Use: Rebinyn® is not indicated for routine prophylaxis or for immune tolerance induction in patients with hemophilia B. |
| Important Safety Information (cont’d) |
| Warnings and Precautions |
| • |
|
Development of neutralizing antibodies (inhibitors) to Factor IX may occur. Monitor patients for development of Factor IX inhibitors if bleeding is not controlled with the recommended dose of Rebinyn® or if expected Factor IX activity plasma levels are not attained. Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used. |
|
| • |
|
The use of Factor IX-containing products has been associated with thrombotic complications. Monitor for thrombotic and consumptive coagulopathy when administering Rebinyn® to patients with liver disease, post-operatively, to newborn infants, or to patients at risk of thrombosis or disseminated intravascular coagulation (DIC). |
|
| • |
|
Nephrotic syndrome has been reported following immune tolerance induction therapy with Factor IX products in hemophilia B patients with Factor IX inhibitors, often with a history of allergic reactions to Factor IX. The safety and efficacy of using Rebinyn® for immune tolerance induction have not been established. |
|
| Adverse Reactions |
| • |
|
The most common adverse reactions reported in clinical trials (≥1%) were itching and injection site reactions. |
|
| • |
|
Animals administered repeat doses of Rebinyn® showed accumulation of PEG in the choroid plexus. The potential clinical implications of these animal findings are unknown. |
|
| Please click here for Prescribing Information. |
|
|
| 1. |
Rebinyn® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; June 2020. |
| 2. |
Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124(26):3880-3886. |
| 3. |
Negrier C, Knobe K, Tiede A, Giangrande P, Møss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118(10):2695-2701. |
| 4. |
Ettingshausen C, Hegemann I, Simpson M, et al. Favorable pharmacokinetics in hemophilia B for nonacog beta pegol versus recombinant factor IX-Fc fusion protein: a randomized trial. Res Pract Thromb Haemost. 2019;3(2):268-276. |
|
|
<%@ include view='hcpColoradoFooter' %>
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1-800-727-6500. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (eg, name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
Rebinyn® is a registered trademark of Novo Nordisk Health Care AG.
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
All other trademarks, registered or unregistered, are the property of their respective owners.
© 2022 Novo Nordisk All rights reserved. US22REB00025 June 2022 |
 |
|
|
|