| Explore your options to see what works for you. |
View online |
|

|
Used to treat, prevent, or reduce the number of bleeding episodes in people with hemophilia B.
See full indication below. |
|
|
|
| Choosing a Hemophilia B Factor Treatment? |
Get Greater Factor Levels
With Rebinyn®. |
When compared to Alprolix® in a study, Rebinyn® achieved
4x greater factor in the bloodstream over time.a |

|
| aPhase 1 trial comparing pharmacokinetics of Rebinyn® with Alprolix®. Based upon a phase 1 study of 15 patients administered a single dose of Rebinyn® 50 IU/kg compared with a single dose of Alprolix® 50 IU/kg using both 1-stage (shown above) and chromogenic assays. The standard Alprolix® dose of 50 IU/kg was administered for both products to allow for comparison of dose-dependent parameters; dose normalized to 50 IU/kg to reflect minor differences in dose administered. Geometric mean half-life was also prolonged (Rebinyn®: 103.2 hours, Alprolix®: 84.9 hours). All comparisons were significant (P<0.0001) for all assays. The clinical relevance of these pharmacokinetic differences is unknown. |
|
|
| In another study, once-weekly Rebinyn® achieved a 115-hour average half-life in adults.b |
| And Rebinyn® elevates factor above your baseline levels: |
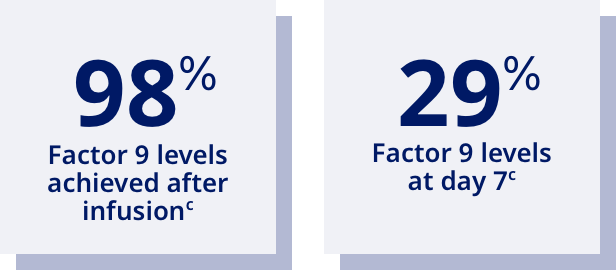 |
| Half-life=Time taken for the level of factor in the blood to fall by 50%. |
| bBased on analysis using a 1-stage assay in patients (n=6) aged 18 and older, the half-life at steady state was 115 hours following once-weekly (40 IU/kg) dosing; in patients (n=3) aged 13 to 17, the half-life at steady state was 103 hours. Following single-dose administration (40 IU/kg) in the same patient population, the half-life was 83 hours (adults) and 89 hours (adolescents). |
| cBased on the mean steady-state post-dose peak levels and pre-dose trough levels 168 hours after administered Rebinyn® 40 IU/kg once weekly in previously treated patients (20 adult and 9 adolescent patients). |
|
| Scroll to view more comparisons. |
 |
|
|
| Selected Important Safety Information |
| What is the most important information I need to know about Rebinyn®? |
| • |
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia treatment center. Carefully follow your healthcare provider’s instructions regarding the dose and schedule for infusing Rebinyn®. |
|
Who should not use Rebinyn®?
Do not use Rebinyn® if you: |
| • |
are allergic to Factor IX or any of the other ingredients of Rebinyn®. |
|
| • |
are allergic to hamster proteins. |
|
| Please click here or scroll below for additional Important Safety Information. |
|
|
| Rebinyn® Delivered Higher Factor 9 Levels Longer Than Alprolix®d |
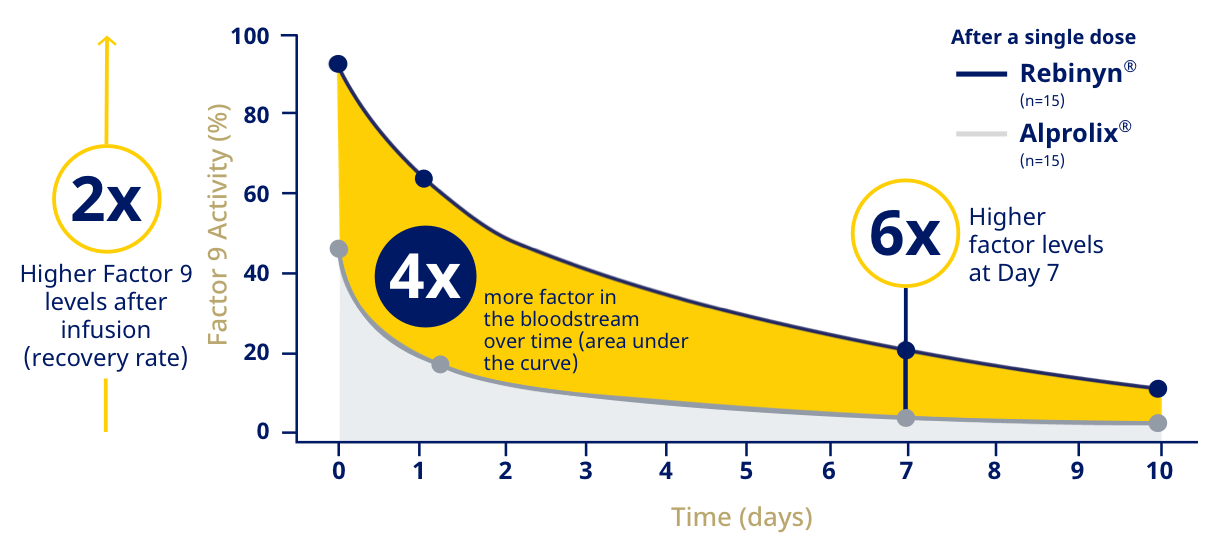 |
| dPhase 1 trial comparing pharmacokinetics of Rebinyn® with Alprolix®. Based upon a phase 1 study of 15 patients administered a single dose of Rebinyn® 50 IU/kg compared with a single dose of Alprolix® 50 IU/kg using both 1-stage (shown above) and chromogenic assays. The standard Alprolix® dose of 50 IU/kg was administered for both products to allow for comparison of dose-dependent parameters; dose normalized to 50 IU/kg to reflect minor differences in dose administered. Geometric mean half-life was also prolonged (Rebinyn®: 103.2 hours, Alprolix®: 84.9 hours). All comparisons were significant (P<0.0001) for all assays. The clinical relevance of these pharmacokinetic differences is unknown. |
|
|
| In a phase 1 study, the half-life of Rebinyn® was 103 hours vs. 85 hours for Alprolix®.e |
|
|
|
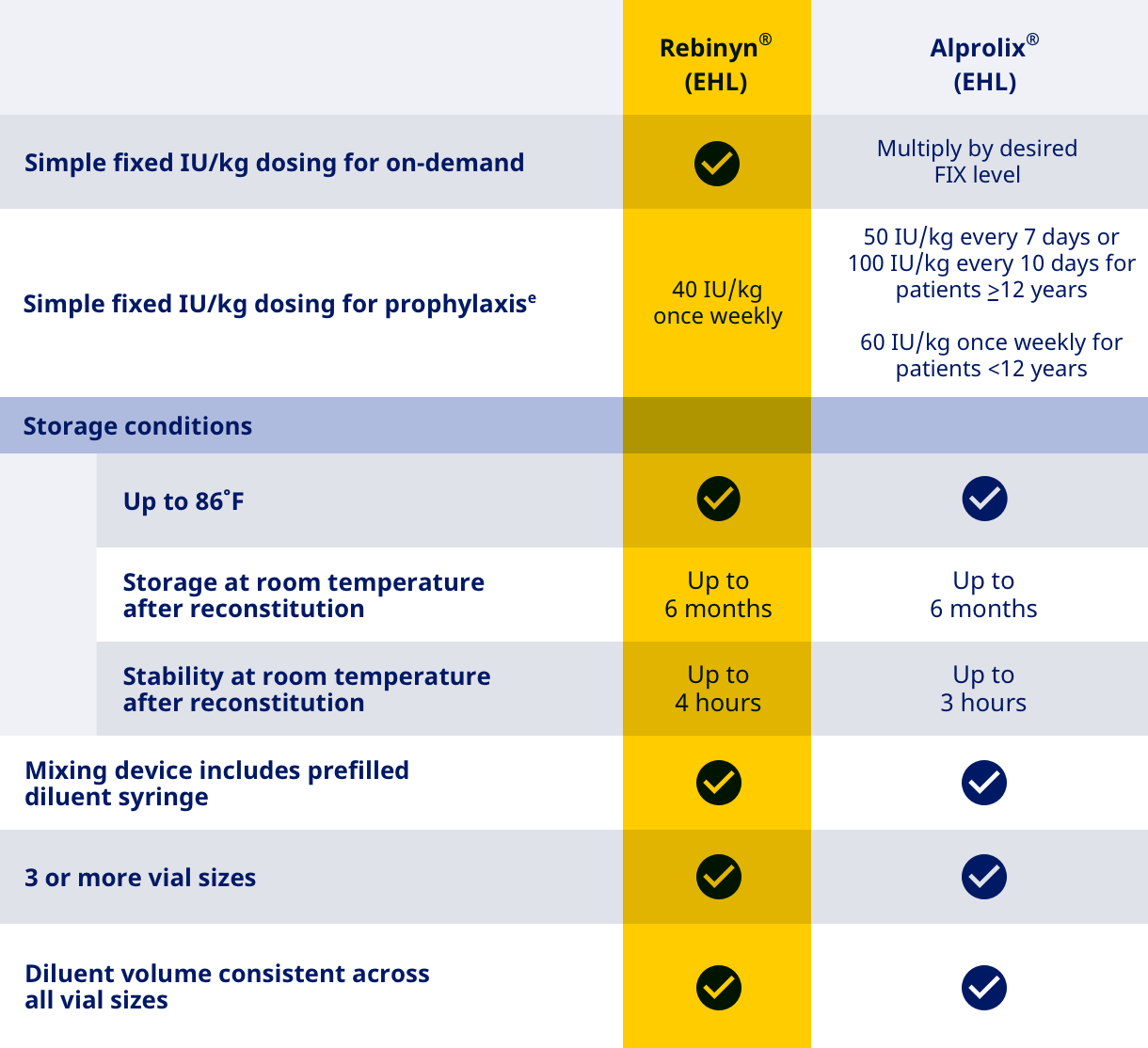
| Here’s how Rebinyn® compares with Alprolix® based on selected key attributes. |
|
| eAdjust dosing regimen based on individual response |
| Data from Rebinyn® PI, 2022; Alprolix® PI, 2020; Idelvion® PI, 2021; BeneFIX® PI, 2021; Ixinity® PI, 2021; and Rixubis® PI, 2020. |
|
|
|
|
| Indications and Usage |
| What is Rebinyn®, Coagulation Factor IX (Recombinant), GlycoPEGylated? |
| Rebinyn® is an injectable medicine used to replace clotting Factor IX that is missing in patients with hemophilia B. Rebinyn® is used to treat, prevent, or reduce the frequency (number) of bleeding episodes in people with hemophilia B. Your healthcare provider may give you Rebinyn® when you have surgery. Rebinyn® is not used for immune tolerance therapy. |
| Important Safety Information |
| What is the most important information I need to know about Rebinyn®? |
| • |
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia treatment center. Carefully follow your healthcare provider’s instructions regarding the dose and schedule for infusing Rebinyn®. |
|
| Who should not use Rebinyn®? |
| Do not use Rebinyn® if you: |
| • |
are allergic to Factor IX or any of the other ingredients of Rebinyn®. |
| • |
are allergic to hamster proteins. |
|
| What should I tell my healthcare provider before using Rebinyn®? |
| Tell your healthcare provider if you: |
| • |
have or have had any medical conditions. |
| • |
take any medicines, including non-prescription medicines and dietary supplements. |
| • |
are nursing, pregnant, or plan to become pregnant. |
| • |
have been told you have inhibitors to Factor IX. |
|
| How should I use Rebinyn®? |
| • |
Rebinyn® is given as an infusion into the vein. |
| • |
Call your healthcare provider right away if your bleeding does not stop after taking Rebinyn®. |
| • |
Do not stop using Rebinyn® without consulting your healthcare provider. |
|
| What are the possible side effects of Rebinyn®? |
| • |
Common side effects include infusion site reaction (bruising, bleeding, swelling, pain, or redness), itching, and rash. |
| • |
Your body can also make antibodies called “inhibitors” against Factor IX, including Rebinyn®, which may stop Rebinyn® from working properly. Your healthcare provider may need to test your blood for inhibitors from time to time. |
| • |
Call your healthcare provider right away or get emergency treatment right away if you get, for example, any of the following signs of an allergic reaction: hives, chest tightness, wheezing, difficulty breathing, and/or swelling of the face. |
| • |
You may be at an increased risk of forming blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider if you have chest pain, difficulty breathing, leg tenderness, or swelling. |
| • |
Animals given repeat doses of Rebinyn® showed Polyethylene Glycol (PEG) in certain cells in the brain. The potential human implications of these animal tests are unknown. |
|
| Please click here for Prescribing Information. |
Rebinyn® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch, or call 1-800-FDA-1088. |
|
|
| Please do not respond to this email. If you would like to contact us, please click here or call 1‑877‑744‑2579. |
| UNSUBSCRIBE NOTICE |
| If you no longer want to receive communications from Novo Nordisk, click here to unsubscribe. You also may call us at 1-877-744-2579 or send us a letter that includes your full contact information (eg, name, email address, phone) to Novo Nordisk, 800 Scudders Mill Road, Plainsboro, New Jersey 08536. To better understand how Novo Nordisk values your privacy, see our Privacy Statement. |
|
Rebinyn® is a registered trademark of Novo Nordisk A/S
Novo Nordisk is a registered trademark of Novo Nordisk A/S.
© 2023 Novo Nordisk All rights reserved. US22REB00198 February 2023 |
 |
|
|
|